Iron, cobalt, and nickel are the main magnetic metals. Some alloys like steel and rare earth metals like neodymium are also very magnetic. Not all metals react to magnets. Knowing which metals are magnetic helps many industries.
Magnetic metals are important in making things, recycling, building, healthcare, and making energy. For example, magnets move metal parts on factory lines. They also help separate metals when recycling. Magnets power electric motors in cars and other vehicles.
Key Takeaways
- Iron, cobalt, and nickel are the main magnetic metals. These metals are used in many industries. They help in manufacturing, recycling, and energy.
- Magnetic metals have unpaired electrons. These electrons line up and make strong magnetic fields. Non-magnetic metals have paired electrons. These electrons cancel out magnetism.
- Rare earth metals like neodymium make very strong magnets. These magnets are used in phones, electric cars, and wind turbines.
- You can test if a metal is magnetic at home. Use a simple magnet to see if it sticks tightly or not.
- Magnetic metals help power everyday devices. They are in motors, speakers, and hard drives. These metals make life easier and help technology work better.
Magnetic Metals

Iron(Fe)
Iron is a very important magnetic metal. Its magnetism comes from how its atoms are arranged. The electrons in iron spin the same way. This creates a strong magnetic field. Iron’s crystal shape makes it tough and heat-resistant. The way iron atoms line up is important for magnetism. If iron gets hotter than 770°C, it stops being magnetic. This is because the atoms stop lining up. Forces between atoms help keep the magnetism strong.
- Iron is ferromagnetic because of its atoms and crystals.
- Its electrons spin the same way, making a magnetic moment.
- Iron’s crystal shapes help it stay strong and resist heat.
- Iron loses magnetism above the Curie temperature.
- Forces between atoms keep the magnetism strong.
Cobalt
Cobalt is also a main magnetic metal. Like iron, cobalt is ferromagnetic. It has three unpaired electrons in its atoms. This helps cobalt make strong magnetic areas. Cobalt’s Curie temperature is 1,115°C. This means it stays magnetic even when hot. Cobalt is used in permanent magnets and magnetic alloys.
| Characteristic | Description/Value |
|---|---|
| Atomic Structure | 3 unpaired electrons in 3d orbitals, enabling strong magnetic domain alignment. |
| Electronic Configuration | [Ar] 3d7 4s2, responsible for magnetism. |
| Magnetic Moment | 2.49 Bohr magnetons (mB), strong ability to align magnetic domains. |
| Curie Temperature | 1,115 °C, maintains ferromagnetism at high temperatures. |
| Magnetic Susceptibility | High, reflecting strong magnetization in external magnetic fields. |
| Magnetic Behavior | Ferromagnetic and permanent magnet, with unique strength and stability. |
| Alloy Formation | Combines with metals like chromium and nickel to form strong magnetic alloys. |
| Grouping | Part of the ‘Iron Triad’ (Fe, Co, Ni), all ferromagnetic. |
Cobalt is mostly found with nickel and copper. The Democratic Republic of the Congo has the most cobalt. Getting cobalt takes many steps, like roasting and refining. Cobalt alloys are used in transformer cores and sensors. More cobalt alloys are needed for electric cars and green energy.
Nickel
Nickel is another main magnetic metal. It is ferromagnetic at room temperature. Nickel has two unpaired electrons in its atoms. Its crystal shape helps it resist rust and gives it medium magnetism. Nickel is used in ships, shields, sensors, and medical tools.
| Property | Nickel | Iron | Cobalt |
|---|---|---|---|
| Magnetic Type | Ferromagnetic at room temperature | Ferromagnetic at room temperature | Ferromagnetic at room temperature |
| Atomic Origin | 2 unpaired electrons in 3d orbital | Unpaired electrons in 3d orbital | Unpaired electrons in 3d orbital |
| Crystal Structure | Face-centered cubic (FCC) | Body-centered cubic (BCC) | Hexagonal close-packed (HCP) |
| Magnetic Saturation | Lower than iron | Highest among the three | Intermediate |
| Magnetic Permeability | Moderate (100-600) | Very high (up to 200,000) | Variable, generally high |
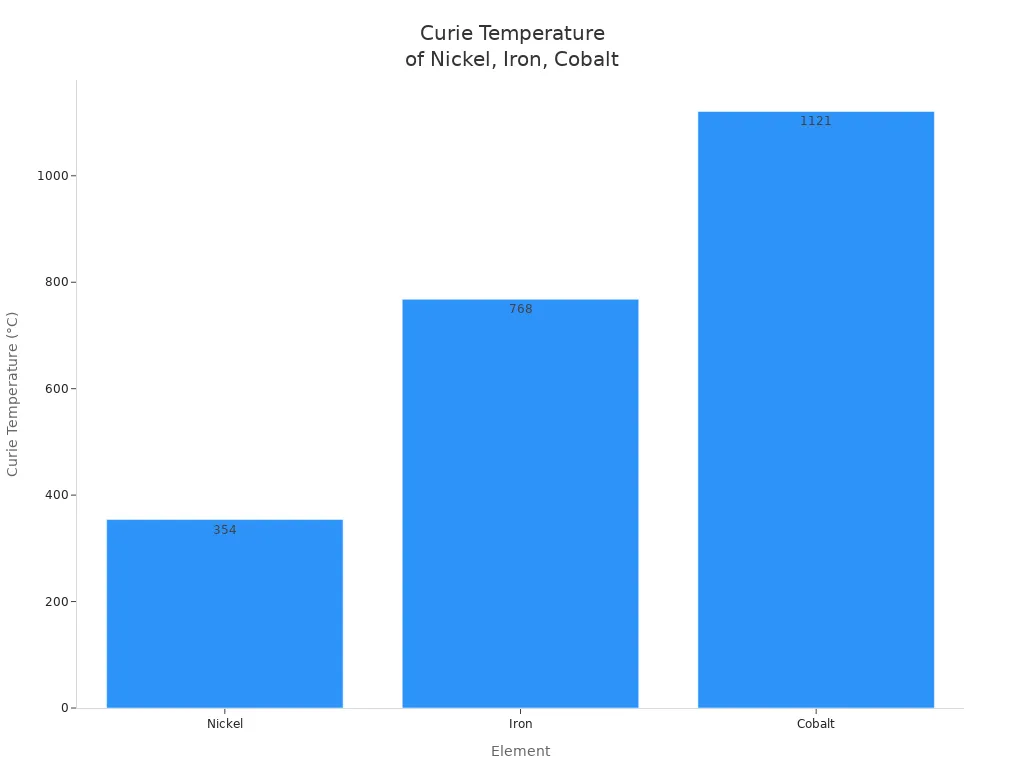
| Curie Temperature | ~354°C (627K) | ~768°C (1043K) | ~1121°C (1394K) |
| Thermal Stability | Moderate | Moderate | Highest among the three |
| Practical Applications | Marine, shielding, sensors, medical devices | Transformers, high magnetic flux | High-temperature applications |
| Corrosion Resistance | High | Lower | Moderate |
Alloys
Many alloys made from magnetic metals are very magnetic. Steel, which has iron, is the most common magnetic alloy. Other alloys include alnico, Inconel, Monel, Permalloy, and Mumetal. These alloys mix metals to make them stronger and better at resisting heat.
| Alloy Name | Typical Composition | Magnetic Properties / Notes |
|---|---|---|
| Neodymium–Iron–Boron (Nd2Fe14B) | Nd, Fe, B | Highest maximum energy product; strong permanent magnets |
| Iron–Platinum Alloys | Fe, Pt | High-performance permanent magnets |
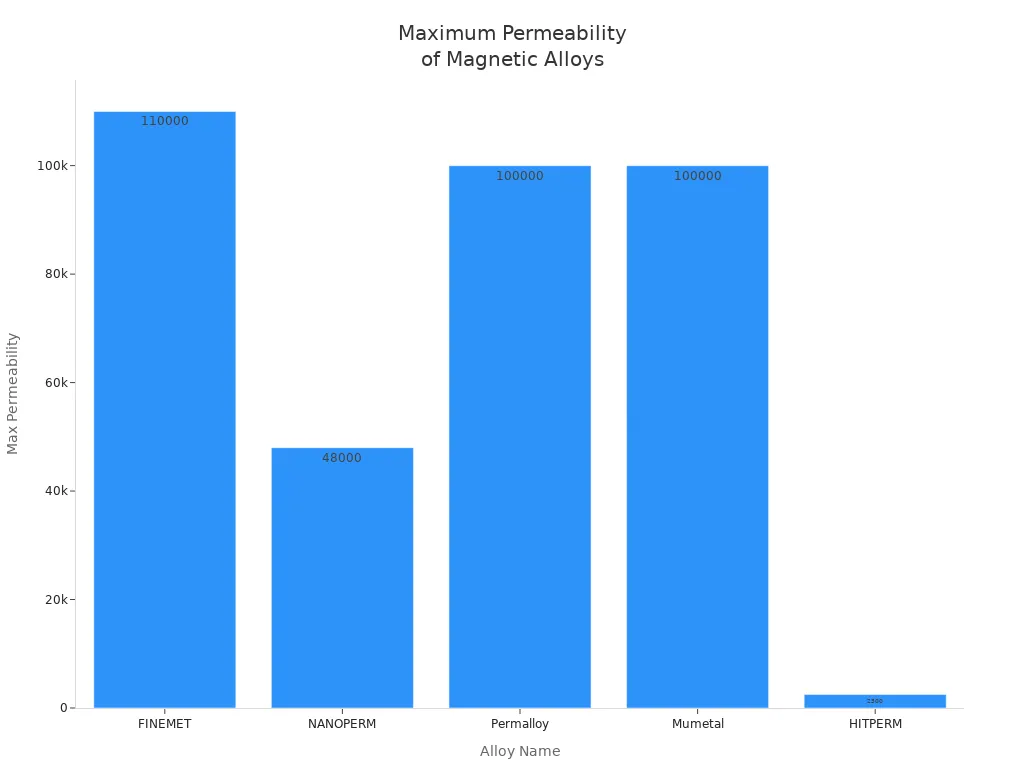
| FINEMET | Fe73.5Cu1Nb3Si13.5B9 | Soft magnetic alloy; very high permeability, low hysteresis loss |
| NANOPERM | Fe86-91Zr7B3-6Cu<1 | Soft magnetic alloy; high permeability, moderate saturation induction |
| HITPERM | Fe44Co44Zr7B4Cu1 | Soft magnetic alloy; permeability 1,000–2,500; saturation induction 1.6–2 T |
| Permalloy | Fe-Ni 70–80% Ni | Very high permeability; used in magnetic shielding and recording heads |
| Mumetal | Fe-Ni ~80% Ni with Cr, Cu, Mo additions | High permeability; used in transformers and magnetic screens |
| Ferrites | MO·Fe2O3 (M = Fe, Ni, Mn, Zn, Co, Cu, Li, Al) |
Ceramic magnetic oxides; high resistivity; used in inductors and transformers |
| Garnets (e.g., Yttrium Iron Garnet) | 3Y2O3·5Fe2O3 with Al, Gd additions | Used in microwave devices; very low loss and high temperature stability |
- Alnico is used for strong magnets in motors, speakers, and sensors.
- Inconel is used in engines and reactors because it resists heat.
- Monel is good for chemical plants, ships, and oil work because it does not rust.
Nickel–iron alloys like Permalloy and Mumetal are best for magnetic shielding. Ferrites and garnets are ceramics with magnetism, used in electronics.
Rare Earth Metals
Rare earth metals like neodymium, dysprosium, and samarium are very magnetic. Their electrons are arranged in a special way. This gives them strong magnetic fields. Rare earth metals are different from other magnetic metals. Pure rare earth metals lose magnetism if they get too cold. So, they are mixed with iron or cobalt to make strong magnets like Nd2Fe14B and SmCo5.
- Neodymium magnets can hold much more energy than iron magnets of the same size.
- Samarium cobalt magnets are a little weaker but can handle heat and do not rust.
- Rare earth magnets are stronger than ferrite or alnico magnets but can break easily and need coatings.
| Feature | Rare Earth Magnets (NdFeB, SmCo) | Regular Magnets (Ferrite, Alnico) |
|---|---|---|
| Magnetic Strength | Very high (up to 1.4 Tesla) | Moderate to low (0.2–0.4 Tesla) |
| Size for Same Strength | Much smaller | Larger for equivalent force |
| Temperature Resistance | SmCo: Excellent; NdFeB: Moderate | Generally good (Ferrite up to ~250°C) |
| Corrosion Resistance | Poor (NdFeB) without coating | Excellent (Ferrite); Alnico also corrosion-resistant |
| Demagnetization Resistance | Excellent (especially SmCo) | Fair to good (Alnico can demagnetize easily) |
| Common Applications | Motors, sensors, MRI, wind turbines | Speakers, fridge magnets, toys |
Neodymium magnets are used in phones, headphones, and hard drives. The neodymium magnet market was worth about $12.5 billion in 2023. It may double by 2032. These magnets are important for wind turbines and electric cars.
Materials with iron, cobalt, nickel, or rare earth elements can be magnetic. Engineers make these materials for things like sensors and motors.
Types of Magnetism

Magnetism comes in different types. Scientists put these types into three groups: ferromagnetism, paramagnetism, and diamagnetism. Each group shows how metals and other things act around magnets.
Ferromagnetism
Ferromagnetism is the strongest kind of magnetism. It is the most important for magnetic metals. In ferromagnetic materials, atoms line up together. Their magnetic moments all point the same way. This makes a strong and lasting magnetic field. Iron, cobalt, and nickel have this property. Some rare earth metals, like gadolinium and dysprosium, can also be ferromagnetic in special cases.
| Defining Characteristics of Ferromagnetism | Metals Exhibiting Ferromagnetism |
|---|---|
| Spontaneous magnetization | Iron (Fe), Cobalt (Co), Nickel (Ni) |
| Hysteresis | Gadolinium (Gd), Dysprosium (Dy) |
| Curie temperature | Alloys of these metals, Heusler alloys |
| Crystal and microstructure dependence | |
| Unpaired electrons in d- or f-orbitals |
Ferromagnetic metals can keep their magnetism after the outside field is gone. They also have a Curie temperature. If they get hotter than this, they lose their magnetism. Most metals used in motors and electronics are ferromagnetic.
Ferromagnetic materials can become magnets on their own. They are strongly pulled by magnets. These materials are used in many modern devices.
Paramagnetism
Paramagnetism is not as strong as ferromagnetism. Paramagnetic materials have unpaired electrons. But their magnetic moments do not stay lined up. When you put them in a magnetic field, they are weakly attracted. When you take away the field, the magnetism goes away.
Some paramagnetic metals are aluminum, titanium, and manganese. Some rare earth elements, like neodymium and dysprosium, are also paramagnetic. Stainless steel and some titanium alloys are in this group too.
- Paramagnetic metals have a small positive magnetic susceptibility.
- They do not keep magnetism when the field is gone.
- Their magnetic response gets stronger when it is colder.
Ferromagnetic metals are much more magnetic than paramagnetic ones. Only ferromagnetic metals can become permanent magnets.
Diamagnetism
Diamagnetism is the weakest type of magnetism. All materials have some diamagnetism, but it is usually hidden by stronger effects. Diamagnetic materials make a tiny magnetic field that goes the other way from the applied field. This causes a weak push away from magnets.
| Material | Magnetic Susceptibility (χv × 10⁻⁵ SI units) |
|---|---|
| Superconductor | −1,050,000 |
| Bismuth | −166 |
| Gold | −28 |
| Silver | −26 |
| Lead | −18 |
| Copper | −10 |
| Water | −9.1 |
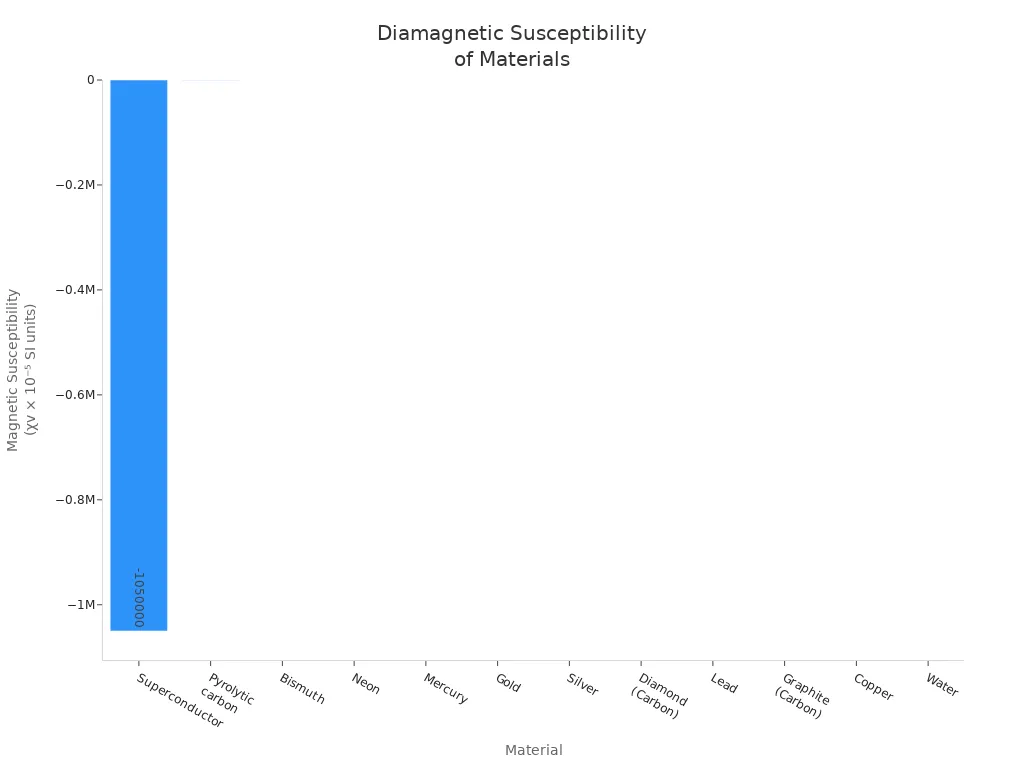
Bismuth is the strongest natural diamagnetic metal. Copper, gold, silver, and lead are also diamagnetic. These metals are weakly pushed away by magnets. They never keep any magnetism. Diamagnetic effects are used in magnetic levitation and shielding.
Diamagnetic materials always push against magnetic fields. Their effect is very small compared to ferromagnetic or paramagnetic materials.
Simple Tests
You can check if a metal is magnetic at home. All you need is a magnet, like one from your fridge. Here’s how you can do it:
- Get a magnet. A fridge magnet works fine.
- Wipe the metal if it is dirty.
- Put the magnet on the metal.
- Try to pull the magnet off.
- If the magnet stays on tight, the metal is probably magnetic.
- If the magnet falls off or comes off easily, the metal is likely not magnetic.
This test works well for most metals, like iron and steel. Some stainless steels might not react, even though they have iron. The magnet test is quick for checking most things at home.
The magnet test helps people sort metals for recycling. It also helps you figure out what a metal object is.
Conclusion
So, to answer the question—what metals are magnetic? The main magnetic metals are iron, nickel, and cobalt, which are ferromagnetic and form the basis of many permanent magnets. Other metals, like aluminum and magnesium, are weakly attracted to magnetic fields but are classified as paramagnetic. Finally, materials like copper, gold, and bismuth are diamagnetic, meaning they are weakly repelled by magnetic fields.
Magnetism is not just a scientific curiosity; it has practical applications in everything from household magnets to advanced technologies like motors, hard drives, and MRI machines. Understanding the magnetic properties of different metals helps engineers and scientists design better products, machines, and tools for everyday use and specialized fields.
FAQ
What makes a metal magnetic?
A metal is magnetic if it has unpaired electrons. These electrons spin the same way. This makes a strong magnetic field. Iron, cobalt, and nickel have this feature.
Can stainless steel be magnetic?
Some stainless steel types are magnetic. If it has more iron and less nickel, it can stick to magnets. Ferritic and martensitic stainless steels are like this. Austenitic stainless steel, like 304 or 316, does not stick to magnets.
Why do magnets not stick to aluminum or copper?
Aluminum and copper have paired electrons. Their atoms do not line up for magnetism. Magnets cannot pull these metals.
Are all alloys with iron magnetic?
Not every iron alloy is magnetic. Other metals in the mix can change things. For example, austenitic stainless steel has iron but does not stick to magnets.
Where do people use magnetic metals in daily life?
People use magnetic metals in motors, speakers, and door latches. They are also in electronic devices. These metals help machines work and keep doors closed. They also store data in computers.

