Magnets are common items in our daily lives, from refrigerator magnets to headphones and electric motors. But have you ever wondered why magnets can attract metals such as iron, nickel, and cobalt, but not copper, aluminum, or plastic? The principle behind this involves the movement of electrons in the microscopic world and the magnetic properties of materials. This article will deeply analyze the scientific mechanism of magnets attracting iron.
1. The origin of magnetism
The origin of magnetism: electron spin and magnetic moment
The magnetism of magnets comes from the movement of electrons inside atoms. Specifically, there are two key factors:
Electron spin: Electrons rotate like a small gyroscope, generating tiny magnetism, called “spin magnetic moment”.
Electron orbital motion: When electrons move around the nucleus, magnetism is also generated, called “orbital magnetic moment”.

In most materials, the directions of these magnetic moments are chaotic and cancel each other out, so the overall magnetism is not apparent. But in ferromagnetic materials (such as iron, nickel, and cobalt), the magnetic moments can spontaneously align to form macroscopic magnetism.
2. Special structure of ferromagnetic materials
Not all atoms in ferromagnetic materials have the same magnetic moment, but are divided into many tiny magnetic domains. The direction of the atomic magnetic moment in each domain is the same.
Unmagnetized: The directions of the magnetic moments of different magnetic domains are randomly arranged, and the material is not magnetic as a whole.
After magnetization: Under the action of an external magnetic field, the directions of the magnetic domains tend to be consistent, and the material exhibits strong magnetism.
When a magnet approaches an iron block, the magnetic domains inside the iron block will gradually align and be attracted by the magnet.
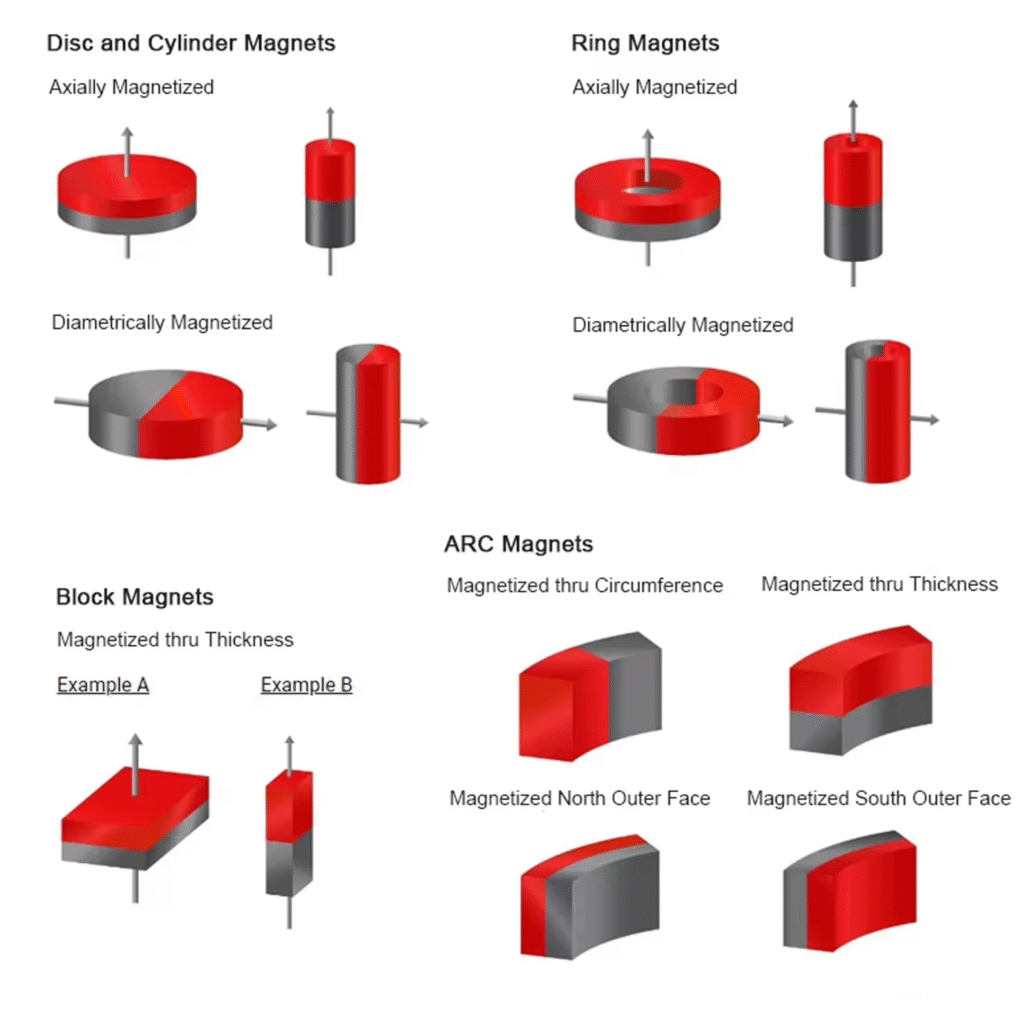
3. Why do magnets only attract metals such as iron, nickel, and cobalt?
Not all metals can be attracted by magnets. Only ferromagnetic materials will exhibit strong magnetism. The reason is:
Iron, nickel, and cobalt: The atomic structure is special, and the electron spins can spontaneously arrange to form stable magnetic domains.
Paramagnetic materials (such as aluminum and oxygen): can be weakly magnetized under an external magnetic field, but cannot maintain magnetism.
Diamagnetic materials (such as copper, water, plastic): will produce reverse magnetism under an external magnetic field, so they will not be attracted.
Interesting phenomenon: Superconductors are completely diamagnetic at low temperatures and can even “levitate” above magnets (quantum locking effect).

4. Can magnets attract all iron products?
Not necessarily! The magnetism of iron products also depends on their microstructure:
Pure iron (soft magnetic material): easy to be magnetized, but also easy to demagnetize (such as iron nails).
Steel (hard magnetic material): contains carbon or other alloy elements, and the magnetic domain is more stable (such as permanent magnets).
Stainless steel: Some stainless steels (such as 304) are austenitic structures and are almost non-magnetic; while magnets may attract martensitic stainless steel (such as steel for knives).
Conclusion
The ability of magnets to attract iron is essentially due to the electron spin and magnetic domain arrangement inside the ferromagnetic material. When the magnet approaches, the direction of the magnetic domain inside the iron block is “tamed”, forming macroscopic magnetism, thereby generating attraction. This principle not only explains everyday phenomena, but also plays a key role in technologies such as motors, magnetic levitation, and medical MRI.
The next time you use a magnet to pick up a paper clip, think about it: behind this seemingly simple action, there is actually an exquisite combination of quantum mechanics and material science!

